Something new under the sun… carboxysome assembly in real time.
Today’s post features research out of the Kerfeld Lab from a close colleague of mine, Jeff Cameron.* After doing his thesis work on redox homeostasis in cyanobacteria, he moved on to a postdoc on the dark side of photosynthesis.** His new paper just out in Cell today sheds new (fluorescent) lights on a longstanding question in cyanobacterial carbon fixation.
Cameron et al. Cell Volume 155, Issue 5 2013 1131 – 1140
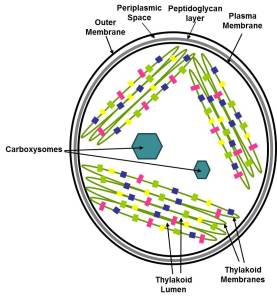
I’ve mentioned before that of the oxygenic photosynthetic organisms, cyanobacteria have an elaborate system for concentrating CO2 at the site of Rubsico. This prevents Rubisco from getting confused and using O2 instead, a costly mistake for the cells. Sure, these cells have an array of transporters as well as carbonic anhydrases to interconvert CO2 and bicarbonate. But the real heart of the cyanobacterial Carbon concentrating mechanism (CCM) is the carboxysome- an intracellular microcompartment where the cells corral all of their Rubsico. This structure allows the cells to handily funnel all of their acquired CO2 so that it can be fixed by Rubisco. No, I didn’t say it’s an organelle. Cyanobacteria are prokaryotes; while they may break the rules by having an internal thylakoid membrane system, they do not have true organelles in the sense that eukaryotes do.
So what are they exactly? They are protein-based structures, in which an array of Rubsico is encapsulated by hexameric and pentameric shell proteins. Carboxysomes have been well-studied from a structural standpoint and the major protein components have been identified.

Schematic model of the α-carboxysome assembly containing RuBisCO small (dark green) and large (green) subunits and carbonic anhydrase (red). The shell is composed of hexamers (blue), pseudohexamers (light blue, magenta, and light green), and pentamers (yellow) From Kinney et al 2011 Photosynthesis Research
However, all of this beautiful structure work doesn’t provide any information on the dynamics of these components as the cells must construct them.
Cameron and co-authors describe an experimental system that allowed them to follow the assembly of carboxysomes from scratch. This system also allowed them to eliminate carboxysome subunits one at a time and monitor the effects on assembly. From all of these results, they were able to piece together a model for carboxysome biogenesis, something that wasn’t possible from looking at static pictures from single mutants.
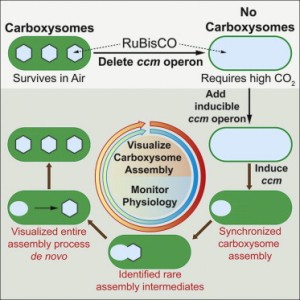
Graphical Abstract
Cameron et al 2013 Cell
Biogenesis of a Bacterial Organelle: The Carboxysome Assembly Pathway
Here’s how the method breaks down:
Observations: Mutants of Synechococcus elongatus PCC 7942 that lack the carboxysome structural proteins cannot confine their Rubisco to small areas and require higher than air-levels of CO2 to grow photosynthetically. Individual mutants of the various carboxysome subunits have variable effects on carboxysome structure, but these are not useful for understanding how the carboxysome subunits come together in a temporal sequence during assembly.
Hypothesis: If a system could be generated to synchronize carboxysome assembly (going from no carboxysomes present to fully assembled carboxysomes), then researchers could probe the effects of the presence/absence of structural components on assembly. This information will allow researchers to deduce the order of assembly events.
Experiment: A synchronized carboxysome assembly system was generated by starting from a mutant of Synechococcus elongatus PCC 7942 lacking all carboxysome structural subunits (no carboxysomes). These structural subunits were added back to this mutant background (as a complete set and as with individual missing subunits) under the control of an inducible promoter. These various cell lines were also engineered to express a fluorescent version of Rubisco were produced to serve as a marker for carboxysome assembly (diffuse fluorescence = no assembly; tight fluorescent spots = assembly).
Results: The control cell line (fluorescent Rubisco + complete set of structural carboxysome subunits under inducible control) showed a diffuse localization of Rubisco throughout the cells prior to the induction of carboxysome genes. After the addition of the inducer chemical, the fluorescence from Rubisco focuses into small green spots indicative of carboxysome assembly. The cases where less-than-the-complete-set of carboxysome subunits were added back to the original mutant cell line, the induction yielded variable results for carboxysome assembly. Some did not assemble any carboxysomes (diffuse Rubisco fluorescence) while others showed intermediate patterns of Rubisco fluorescence (abnormal or partially assembled carboxysomes).
Conclusions: The authors deduced a new model for carboxysome assembly. First Rubisco proteins and the subunit CcmM aggregate together to for pro-carboxysomes (PCs). CcmN is then added to the PCs and begins to associate with the outer shell proteins CcmK2 and CcmO. Finally, when the CcmL protein is incorporated, the carboxysome completely enclosed by its protein shell and released from the PC.
Think Ahead: Carboxysomes are a prototype for other bacterial microcompartments. Other bacteria use similar protein-based microcompartments for specialized metabolism. This system and the insights on the timing sequence of its assembly can applied to these other systems. In any case, these experiments lay the groundwork for engineering similar microcompartments for medical or biotech purposes. For instance, plants might be coerced to make carboxysomes for more efficient carbon fixation. Alternatively, it may be possible to create novel microcompartments in other bacteria for the purposes of efficiently making useful metabolites.
Check out the link below for the fluorescence microscopy video of carboxysome biogenesis. Sorry, there’s a paywall for 12 months.
UPDATE: There is a free version of Supplemental Video #2. Thanks JGI!
This is after all carboxysome genes have been turned on and you can watch them move and even see some bud off after awhile. (I still prefer the other video where you can watch them form from nothing.) Also, in the current edition of the video caption, they credit James Cameron instead of Jeff Cameron. Sure, James does great cinematic work- just not this one. (It should be fixed on Monday.)
Jeff Cameron, the James Cameron of carboxysome cinema.
Johnna
*Jeff and I were graduate students together in Himadri Pakrasi’s lab at WashU. I think he still hears my voice in his head when he does biochemical purifications. “Fast and Cold! Jeff. Fast. And. Cold.”
**Yes, yes, I know. Light-independent. Whatever. ‘Dark’ makes for catchier writing and storytelling.
References:
http://www.sciencedirect.com/science/article/pii/S0092867413013627
http://link.springer.com/article/10.1007%2Fs11120-011-9624-6/fulltext.html